A couple of days in the Lake District helping Martyn with fieldwork has offered a veritable smorgasbord of plants this week, several new to me, so where to start?
What about with those plants which give animals a run for their money, ‘eating’ insects to boost the levels of nitrogen available to them in nutrient poor environments? Obtaining nitrogen like this is an expensive business for plants; they need to attract suitable insects in some way, build specialised structures to capture them and then produce the enzymes necessary to break them down into a form which can be taken up and used by the plant.
I’ve written about these fascinating plants before. They tend to grow in waterlogged environments where the recycling of mineral nutrients from decaying plant and animal material is limited by a lack of oxygen, so their options are limited. We often think of them as tropical plants – Venus fly traps or Pitcher plants, for example – but there are around a dozen species native to the UK. Charles Darwin was the first person to confirm that the Sundews he found on Sussex heathland not far from his home at Down House were using insects to meet their nutritional needs. Darwin’s book ‘Insectivorous Plants’, published in 1875, is a fascinating account of his experiments feeding all kinds of things, including raw meat and bits of hard boiled egg, to the plants and making the kind of detailed observations of their responses which you might expect of him.
I found three different insectivorous plants around Wastwater, one of the more remote lakes in the south west of the Lake District. These included two of our three native species of Sundew, which catch insects by means of sticky glands on their highly-specialised leaves. Glands on long stalks (tentacles) around the edge of the leaf first attract and trap unwary insects with sweet, sticky mucilage (the ‘dew’), then secrete enzymes to break down the carcass. It’s not just Venus fly traps which move to capture their prey though – Darwin observed that a gnat’s leg touching a single tentacle was enough to make the surrounding tentacles bend towards the centre of the leaf, bringing the insect into contact with as many sticky glands as possible within an hour or two of that first contact. The more the hapless insect struggles, the faster its engulfment takes place. The process depends on both electrical and chemical signalling in the plant.

First the insect’s struggles generate an electrical signal in the tentacles, which start to bend and to accumulate jasmonates – chemical signalling molecules more normally associated with triggering the response of plants to attack by herbivores. These jasmonates, in turn stimulate the secretion of digestive enzymes and this is enhanced if chemical signals such as chitins and ammonia are picked up from the trapped insect (the red arrows in the figure below). The mixture of enzymes and acids will kill the insect within about 15 minutes before starting, over the next few days, to break it down. The simple molecules produced by the enzymes are then absorbed into the leaf via another set of unstalked glands on the leaf surface to boost the plant’s nutrient status and enhance its ability to photosynthesise.
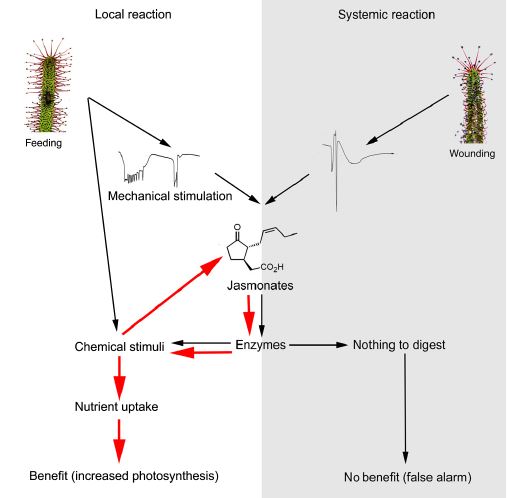
The involvement of jasmonates is strong evidence that carnivory evolved from plants’ need to defend themselves from insect attack. If the leaves are wounded mechanically to mimic a herbivorous attack, systemic electrical signals are produced, throughout the whole plant and jasmonates accumulate in the all leaves, triggering the expression of digestive enzymes. This seems a bit of a waste but is more evidence of the link between the pathways and, without the positive feedback loop shown above, the amount of enzyme produced will be limited.

I found the Sundews on boggy ground just above the lake, growing on water-saturated peat, as well as higher up on acidic flushes on the crags to the east of Wastwater and again around Haweswater. Low temperatures, as well as a lack of oxygen, may be reducing nutrient cycling in these habitats.
The third insectivorous plant I found was Butterwort, Pinguicula vulgaris, which has less obviously sticky leaves though they act equally efficiently, like fly paper, to trap small insects such as fungus gnats. Pinguicula can also trap and consume small bits of plant material such as pollen and leaves and has invested energy which might otherwise be spent on root development in producing large, specialised leaves to secure its nitrogen supply. We found Butterwort growing high up on the fells to the east of Wastwater, on the banks of Ennerdale Water and above Haweswater.

Some of the Butterwort leaf glands are on stalks and these secrete mucilage and digestive enzymes in the same was as the Sundew glands, making the leaf surface appear shiny. Sessile glands sitting directly on the leaf surface are the main source of digestive enzymes, though. Insect bodies are broken down where they lie by the secreted enzymes and the resultant nutritious soup is then absorbed through pores in the cuticle, leaving the indigestible chitin exoskeleton behind.
The Sundews we found were still in bud rather than in flower – both species produce spikes of small, white flowers held on long stalks, well above the leaves. Butterwort flowers are also on long stalks, making it frustratingly difficult to photograph the flower and leaves together! The reason quickly becomes apparent when you realise the flowers rely on insects to pollinate them – the plant needs a way of keeping those insects it requires for pollination safely away from its dangerous leaves.
A 2016 paper by El-Sayed and colleagues throws some light on how plants manage this, showing with a series of clever experiments that insectivorous plants rely variously on visual, chemical and spatial cues to spare pollinating insects. In most of the Drosera species they looked at, they found that white was more attractive to pollinators and red to other insects and that this, together with the flowers being held well above the leaves, helped reduce the chances of pollinating insects being trapped. In other species, flowers and leaves produced different blends of volatile organic compounds, each designed to attract different groups of insects.
All these adaptations require a significant investment on the part of the plant. As a trade off, both Sundews and Butterworts invest very limited resources in root growth – after all, a large root area is not needed to take up water in these damp habitats. The extent of the adaptations needed only highlights the importance of plants being able to secure an adequate supply of nitrogen.
Darwin C. (1875) Insectivorous Plants. http://darwin-online.org.uk/.
El-Sayed A.M. et al. (2016) Pollinator-prey conflicts in carnivorous plants: When flower and trap properties mean life or death. Sci. Rep. 6, 21065; doi: 10.1038/srep21065
Krausko M. et al. (2016) The role of electrical and jasmonate signalling in the recognition of captured prey in the carnivorous sundew plant Drosera capensis. New Phytol. 213, pp 1818–1835; doi: 10.1111/nph.14352
I’d always thought of pitcher plants as tropical until we found them growing in the Tablelands in western Newfoundland! https://flic.kr/p/2bUz5Xs
[…] are fascinating plants well adapted to life in nutrient-poor environments (see “It’s a plant eat insect world out there …”) and their presence here made me wonder what else might be growing within this brown […]
[…] be absorbed through the plant leaves (Spomer, 1999). Truly carnivorous plants, such as our native Sundews and Butterworts, invest a lot of resources into obtaining their nitrogen in this way; many questions still remain […]